White Powder Assay 99.5% Min Estrogen Steroids Tamoxifen Nolvadex CAS No 10540-29-1
- Fabricant: Dewael
- Essai de produit: 99%+
- Apparence:poudre cristalline blanche.
- Lieu d'origine: Chine
- certificat: SGS,ISO9001, GMP
- Quantité minimum d'achat: 10g
- Détails de l'' emballage: des moyens discrets d'emballage pour la douane passer garanti
- Heure de livraison: Dans 12 heures après réception de votre paiement
- Modalités de paiement: Western union, MoneyGram,Bitcoin,Virement
- livraison: EMS,DHL,Fedex,UPS,TNT et ainsi de suite.
- Politique: Re-Politique de livraison
- Capacité d'approvisionnement: 500-600kg/mois
White Powder Assay 99.5% Min Estrogen Steroids Tamoxifen Nolvadex CAS No 10540-29-1
Détails rapides :
Nom du produit: Tamoxifène
Alias: Nolvadex-D;tamoxifen(z)
Trade names: Nolvadex, Istubal, Valodex, Genox
CAS Registry Number: 10540-29-1
EINECS Non: 234-118-0
Pureté: 99%
MF: C26H29NO
MW: 371.51
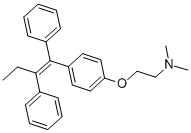
Personnage: Poudre cristalline blanche.
Usage: For the treatment of advanced, recurrent breast cancer and ovarian cancer and other illnesses. Used as an antineoplastic raw materials.
La description :
Tamoxifen is a nonsteroidal anti-estrogen drug. The structures are similar to those of estrogens, and are present in type Z and type O isoforms. The physical and chemical properties are different from each other, and their physiological activities are different. Type E has weak estrogenic activity, and type Z has an anti estrogenic effect. If estrogen receptor (ER) is present in breast cancer cells, estrogen enters tumor cells and combines with ER to promote tumor cell mRNA and DNA synthesis and stimulate tumor cell growth. toutefois, the tamoxifen Z isoform enters the cell and competes with ER to form a receptor complex that inhibits estrogen and inhibits proliferation of breast cancer cells.
Mainly used for the treatment of breast cancer patients with high levels of estrogen, androgen and other anticancer drugs (such as more flexible than the magnitude) combined to improve postmenopausal advanced breast cancer patients after curative effect and shows good curative effect.
Applications :
Tamoxifen is an antagonist of the estrogen receptor in breast tissue via its active metabolite, 4-hydroxytamoxifen. In other tissues such as the endometrium, it behaves as an agonist, and thus may be characterized as a Selective estrogen-receptor modulator. Tamoxifen is the usual endocrine (anti-estrogen) therapy for hormone receptor-positive breast cancer in pre-menopausal women, and is also a standard in post-menopausal women although aromatase inhibitors are also frequently used in that setting.
Tamoxifen is used to treat infertility in women with anovulatory disorders. A dose of 10-40 mg per day is administered in days 3-7 of a woman’s cycle.In addition, a rare condition occasionally treated with tamoxifen is retroperitoneal fibrosis. Tamoxifen is currently used for the treatment of both early and advanced ER+ (estrogen receptor positive) breast cancer in pre- and post-menopausal women. En plus, it is the most common hormone treatment for male breast cancer.It is also approved by the FDA for the prevention of breast cancer in women at high risk of developing the disease.It has been further approved for the reduction of contralateral (in the opposite breast) cancer.
Tamoxifen also increase the risk of adverse side effects. Some cases of lower-limb lymphedema have been associated with the use of tamoxifen, due to the blood clots and deep vein thrombosis (DVT) that can be caused by this medication. Resolution of the blood clots or DVT is needed before lymphedema treatment can be initiated.
COA:
| Éléments de test | spécification | Résultats des tests |
| La description | Poudre cristalline blanche | Poudre cristalline blanche |
| Identification | ET , UV conform | Se conformer |
| Perte au séchage | ≤0.5ml | 0.21ml |
| Résidus au feu | Not more than 0.2% | 0.06ml |
| Iron | Not more than 0.005% | 0.0018% |
| Métaux lourds | Not more than 0.001% | 0.0006% |
| Substances apparentées | Total: Not more than 1.0%
Individual: Not more than 0.5% |
0.32%
0.14% |
| E-isomer | Not more than 0.3% | 0.19% |
| Organic volatile impurities | Conforme | Conforme |
| Essai | 99.0~101,0% | 99.51% |
| Conclusion | Conform with USP30 | |
———————————————————————————————————————————————————————————————————————————————————————-
Contacter maintenant


































Avis
Il n'y a pas encore de critiques.