99% Pure Medical Drug Tadalafil CAS 171596-29-5 Raw Steroid Powders For Male Erectile Dysfunction
- Fabricant: Dewael
- Essai de produit: 99%+
- Apparence:poudre cristalline blanche.
- Lieu d'origine: Chine
- certificat: SGS,ISO9001, GMP
- Quantité minimum d'achat: 10g
- Détails de l'' emballage: des moyens discrets d'emballage pour la douane passer garanti
- Heure de livraison: Dans 12 heures après réception de votre paiement
- Modalités de paiement: Western union, MoneyGram,Bitcoin,Virement
- livraison: EMS,DHL,Fedex,UPS,TNT et ainsi de suite.
- Politique: Re-Politique de livraison
- Capacité d'approvisionnement: 500-600kg/mois
99% Pure Medical Drug Tadalafil CAS 171596-29-5 Raw Steroid Powders For Male Erectile Dysfunction
Détail rapide:
| Nom du produit | Tadalafil |
| Autre nom | cialis; chinacialis |
| Numéro de registre CAS | 171596-29-5 |
| EINECS | — |
| Formule moléculaire | C22H19N3O4 |
| Poids moléculaire | 389.341 |
| Structure moléculaire | 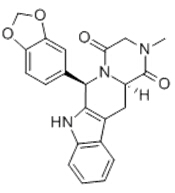 |
| Essai | 99% |
| Apparence | poudre blanche |
| Emballer | 1kg/aluminium foil bag or as required |
| Usage | can be used as pharmaceutical material |
La description:
Cialis (Tadalifil), a phosphodiesterase type 5 inhibitor, is used to treat erectile dysfunction (ED) and decreased libido in males. Cialis works by aiding relaxation of blood vessels and increasing blood flow in the penis during sexual arousal, resulting in improved erectile function.
Tadalafil is used for Erectile dysfunction and pulmonary hypertension, the rapid absorption after oral administration, 2 hours to reach maximum observed plasma concentrations of average time after administration of (Cmax). Influence of absorption rate and degree is not affected by food tadalafil, so it can be with or without with clothes and food.
Tadalafil is an oral drug for the treatment of ED, used in the treatment of erectile dysfunction and premature ejaculation, erectile function impairment and premature ejaculation has very significant improvements. There are over 1500 documents to prove the caused by different causes impotence premature ejaculation, knew the success rate is above 80%, and show its reliable curative effect, through the use of more than 20 million people worldwide, proved its long-term stability, the safety of 25 à 60 minutes of work function to coincide with a time needed for foreplay, knew the time adjustment in the highest drug concentration time, help both husband and wife a satisfactory sex life.
Applications:
Since people who have taken tadalafil within the past 48 hours cannot take organic nitrates to relieve angina (such as glyceryl trinitrate spray), these patients should seek immediate medical attention if they experience anginal chest pain.In the event of a medical emergency, paramedics and medical personnel should be notified of any recent doses of tadalafil.
Tadalafil is metabolized predominantly by the hepatic CYP3A4 enzyme system. The presence of other drugs which induce this system can shorten tadalafil half-life and reduce serum levels, and hence efficacy, of the drug.
Tadalafil has been used in approximately 15,000 men participating in clinical trials, and over eight million men worldwide (primarily in the post-approval/post-marketing setting). The most common side effects when using tadalafil are headache, stomach discomfort or pain, indigestion, burping, acid reflux, back pain, muscle aches, flushing, and stuffy or runny nose. These side effects reflect the ability of PDE5 inhibition to cause vasodilation (cause blood vessels to widen), and usually go away after a few hours. Back pain and muscle aches can occur 12 à 24 hours after taking the drug, and the symptom usually disappears after 48 heures.
COA:
| Éléments de test | spécification | Résultats des tests |
| Apparence | poudre blanche | Conforme |
| Test method | High pressure liquid chromatography(HPLC) | Conforme |
| Point de fusion | 300℃~303℃ | 301.5℃-302.6℃ |
| Perte au séchage | ≤0,5% | 0.24% |
| Métaux lourds(Pb) | ≤20ppm | 7.5ppm |
| Résidus au feu | ≤0,1% | 0.08% |
| Relative substance | ≤1,0% | 0.145% |
| Essai (on dried basis) | ≥98.5% | 99.33% |
| Conclusion | The specification conform to USP 35 | |
HPLC:
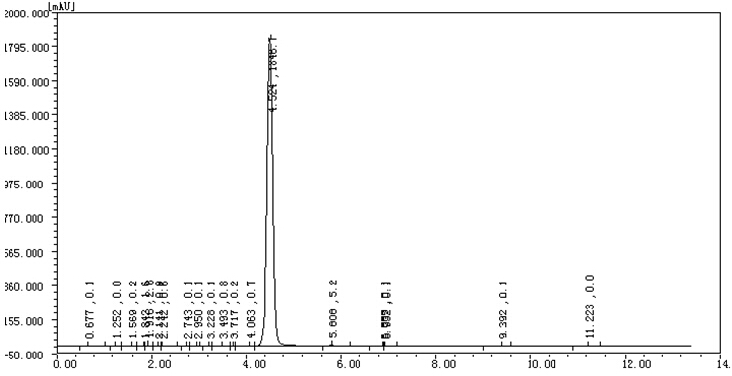
———————————————————————————————————————————————————————————————————————————————————————-
Contacter maintenant


































Avis
Il n'y a pas encore de critiques.